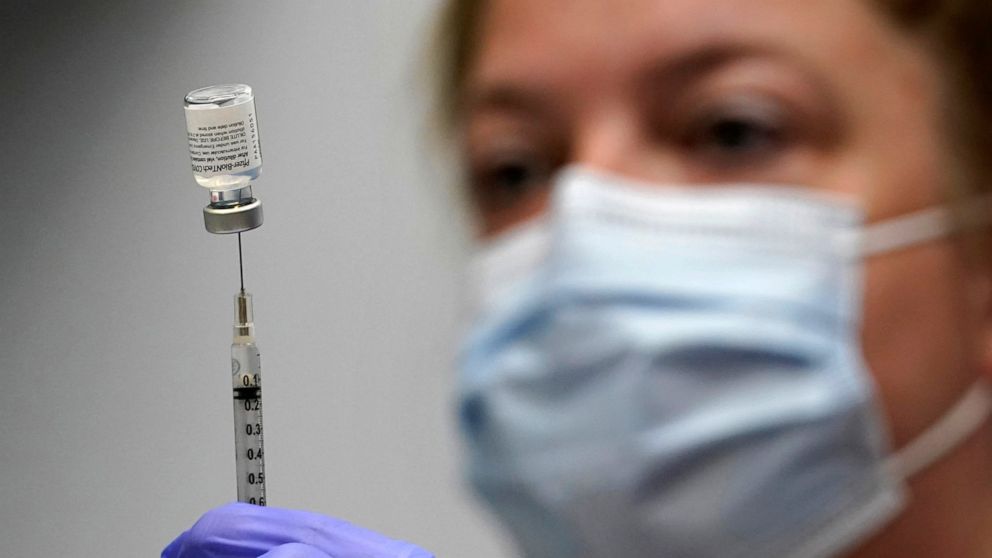
The U.S. Food and Drug Administration (FDA) panel overwhelmingly voted against the Biden administration’s plan to implement booster shots of Pfizer’s COVID-19 vaccines for all Americans on Friday.
In a vote of 16 to two, after reviewing data from the United States, the U.K., and Israel, the FDA’s Vaccines and Related Biological Products Advisory Committee agreed that despite the Biden administration’s push to offer extra shots to people as young as 16 years old in the next few days, there was not enough evidence to support COVID-19 booster shots for every age group.
The panel, however, unanimously voted to give emergency approval to Pfizer booster shots “at least six months following the second dose among people ages 65 and older and those at high risk of occupational exposure and severe COVID-19,” Fox News reported.
The Biden administration previously planned to move forward with supplemental jabs for adults beginning as early as the White House’s Sept. 20 deadline, pending the FDA’s approval. This pressure from the Democrat president and his administration to offer “premature and unnecessary” consent to something that scientific data does not conclusively back up, reports indicated, caused strife within the regulatory agency and even pushed several high-profile FDA officials to resign.
Later, these officials publicly disagreed with the administration’s booster shot push by signing a letter opposing it. --->READ MORE HERE
 |
| The Associated Press |
Dealing the White House a stinging setback, a government advisory panel overwhelmingly rejected a plan Friday to give Pfizer COVID-19 booster shots across the board, and instead endorsed the extra vaccine dose only for those who are 65 or older or run a high risk of severe disease.
The twin votes represented a heavy blow to the Biden administration's sweeping effort, announced a month ago, to shore up nearly all Americans' protection amid the spread of the highly contagious delta variant.
The nonbinding recommendation — from an influential committee of outside experts who advise the Food and Drug Administration — is not the last word. The FDA will consider the group’s advice and make its own decision, probably within days. And the Centers for Disease Control and Prevention is set to weigh in next week.
In a surprising turn, the advisory panel rejected, 16-2, boosters for almost everyone. Members cited a lack of safety data on extra doses and also raised doubts about the value of mass boosters, rather than ones targeted to specific groups.
Then, in an 18-0 vote, it endorsed extra shots for people 65 and older and those at risk of serious disease. Panel members also agreed that health workers and others who run a high risk of being exposed to the virus on the job should get boosters, too. --->READ MORE HEREFollow links below to related stories and resources:
Biden’s playing deadly games with Florida
Making kids under 6 mask isn’t science, it’s child abuse
USA TODAY: Coronavirus Updates
WSJ: Coronavirus Live Updates
YAHOO NEWS: Coronavirus Live Updates
NEW YORK POST: Coronavirus The Latest
If you like what you see, please "Like" us on Facebook either here or here. Please follow us on Twitter here.

No comments:
Post a Comment